What is Evusheld?
Evusheld (designated in the study as AZD7442) is a monoclonal antibody mixture made up of 2 long-acting monoclonal antibodies tixagevimab (AZD8895) and cilgavimab (AZD1061). Currently, Evusheld has been granted a special import license by the Ministry of Health in Vietnam to meet the need for pre-exposure prophylaxis (prevention) of Covid-19 for high-risk subjects such as immunocompromised or unvaccinated people.
Evusheld has been optimized with proprietary technology, which prolongs the half-life by more than 3 times. At the same time, this change also helps the drug to concentrate in high concentration in the nasopharyngeal mucosa and reduce the risk of immune-related diseases such as antibody-dependent disease (antibody-dependent disease).
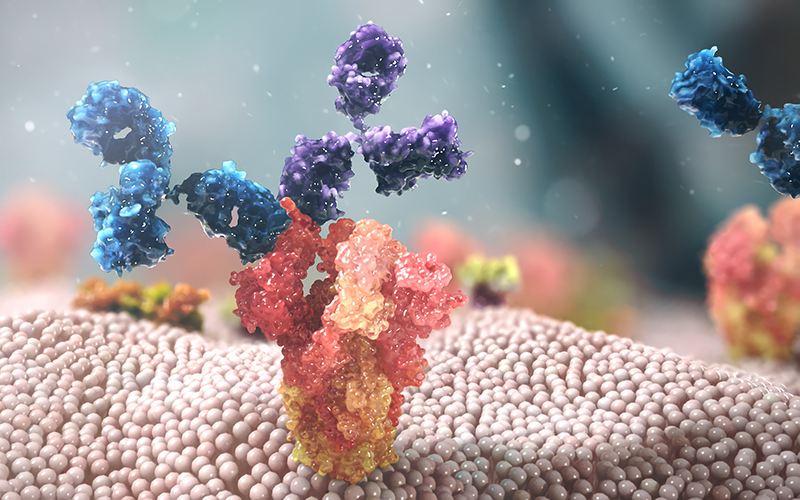
Mechanism of action of Evusheld
Evusheld has a combination of 2 antibodies: tixagevimab and cilgavimab. These two antibodies bind to 2 separate, non-overlapping sites on the spike protein of the SARS-CoV-2 virus, helping to prevent the entry of the SARS-CoV-2 virus into the host cell (preventing the infection). for spike protein to interact with the ACE2 receptor on the cell membrane, resulting in the SARS-CoV-2 virus not being able to enter human cells). The combination of 2 antibodies at the same time brings a synergistic effect and minimizes the risk of resistance of SARS-CoV-2 virus variants.
Why should you inject Evosheld?
According to statistics, about 2% of the global population is classified as a high-risk group that cannot produce an adequate immune response to the Covid-19 vaccine, that is, a group of people with a history of severe allergies to other substances. Covid-19 vaccine, congenital immunodeficiency or HIV, undergoing chemotherapy or radiotherapy for cancer, have a joint disease such as rheumatoid arthritis, osteoarthritis being treated with biomodulators, organ transplant recipients and are being treated with immunosuppressive drugs to prevent transplant rejection or those with systemic diseases being treated with immunosuppressive drugs such as high-dose corticosteroids...,
Monoclonal antibodies are therapeutic important measures against Covid-19. The drug is expected to quickly neutralize and treat Covid-19 for more people, especially the vulnerable, providing long-lasting protection, optimized for protection for at least 6 months. month after only one intramuscular injection. Currently, Evosheld has been licensed in the US, France and a number of other countries.
Myron J. Levin – Professor of Pediatrics and Medicine, University of Colorado School of Medicine (USA) said: “Millions of people in the US and around the world are still at serious risk from Covid-19 because Covid-19 because their immune system does not produce an adequate immune response, even after receiving all the recommended doses of the Covid-19 vaccine. The Evosheld is a new option that is easy to use and provides the long-term protection needed to help people return to normal everyday life."
What are the clinical test results of the Evosheld antibody?
On November 18, 2021, AstraZeneca released new analyzes in two phase III clinical trials showing that:
- According to results from the phase III trial PROVENT (for pre-exposure prophylaxis) involving 5,197 people, AZD7442 resulted in a 77% reduction in the risk of symptomatic Covid-19 compared with placebo in the primary analysis and 83% in the 6-month follow-up analysis.
- The separate TACKLE, phase III outpatient trial (n=903) demonstrated AZD7442 to reduce the risk of developing mild-moderate Covid-19 into severe illness or death (regardless of cause). Compared with placebo, there was a 50% reduction in the risk of severe disease progression or death in patients receiving AZD74442 within 7 days, a 67% reduction if the patient took it within 5 days, and an 88% reduction in the above risk if patients used within 3 days of symptom onset.
The latest preclinical data also show that the antibody duo maintains neutralizing efficacy against the Omicron variant, which is a faster-spreading variant that currently dominates and causes concern worldwide. bridge. In particular, after 6 months of follow-up in the prevention trial, AZD7442 reduced the risk of symptomatic Covid-19 by 83%, and no serious illness or death was recorded in the AZD7442 group.
Professor Hugh Montgomery, Major in Critical Care Medicine at University College London, UK said: “It is important that after 6 months, despite the increase in the Delta variant, the Omicron protective effect of AZD7442 is maintained in people who are at high risk and likely not to produce an adequate immune response to the Covid-19 vaccine.”